
DIAGNOSIS AND TREATMENT
The discovery of pathogenic autoantibodies to voltage-gated calcium channels (VGCCs) has facilitated diagnosis.
—Titulaer et al, 20114
The discovery of pathogenic autoantibodies to voltage-gated calcium channels (VGCCs) has facilitated diagnosis.
—Titulaer et al, 20114
LEMS Testing and Diagnosis
The discovery that P/Q-type anti-VGCC antibodies are a pathogenic factor in Lambert-Eaton myasthenic syndrome (LEMS) represented a major scientific breakthrough. With an established etiological basis for LEMS, physicians now have confirmatory tests and tools to shorten the diagnostic delay.4
Early diagnosis allows for effective treatment of symptoms and an early initiation of oncological treatment, if necessary.4
DIAGNOSTIC CRITERIA AND TESTING
Key LEMS signs and symptoms to watch for during a patient history and initial physical exam4
Start with a patient history
- A history of tobacco smoking is a risk factor for cancer-associated LEMS12
- A history of autoimmune disease is sometimes present in patients with LEMS not associated with cancer4,9,12
Probe for autonomic symptoms
- Dry mouth, oculobulbar weakness, and impotence are strong indicators of LEMS4
Test the patient’s tendon reflexes
- Hyporeflexia and areflexia are distinguishing signs of LEMS4
Ask the patient to rise from a chair
- Proximal leg muscle weakness is a hallmark sign of LEMS4

A diagnosis of LEMS may be suspected based on clinical symptomatology, including proximal muscle weakness, autonomic dysfunction, and hyporeflexia or areflexia. However, confirmatory tests are critical for a definitive diagnosis.4
Tests that can confirm a diagnosis of LEMS
If clinical examination and family history lead you to suspect LEMS, confirmatory diagnosis may be made using one or both of the following test methods:
Anti-VGCC antibody testing
A blood test that measures for elevated levels of VGCC antibodies.4
Electrodiagnostic testing
A nerve stimulation test that measures a muscle’s response to
electric pulses 4
Learn more about these 2 tests below.
ANTI-VGCC ANTIBODY TESTING
Sensitivity for LEMS
Elevated serum titers of autoantibodies to VGCC are found in4,19,20:
- virtually all patients with small cell lung cancer–associated LEMS and are invariably present in patients with other forms of cancer-associated LEMS
- ~90% of patients with LEMS not associated with cancer
Clinical significance
A pathogenic role for anti-VGCC antibodies has been established in LEMS in which autoantibodies target VGCC on the presynaptic nerve terminal of the neuromuscular junction and impair neurotransmission of acetylcholine.4,20
Interpreting results
>30 pmol/L* indicates that the patient is positive for LEMS21
- <30 pmol/L is considered to be a negative test result† and a normal titer level; however:
- A negative test result does not rule out a diagnosis of LEMS†
- Electrodiagnostic testing should be performed as a follow-up1
*30 pmol/L = 0.03 nmol/L. Reference ranges may vary based on specific laboratories.
†A negative anti-VGCC titer does not exclude LEMS; up to 15% of patients with LEMS have undetectable anti-VGCC antibody levels.4
Insights from a LEMS expert
Mazen Dimachkie, MD, FAAN, FANA, a Professor of
Neurology at The University of Kansas Medical
Center, discusses one often-misdiagnosed cause of
weakness and fatigue: LEMS.
Within this video presentation, Dr. Dimachkie walks
through determining the cause of a patient’s muscle
weakness, common clinical symptoms, and
diagnostic testing in order to help clinicians
diagnose LEMS. Excerpts from this presentation
appear throughout this website, and you can watch the full webinar here.*
ELECTRODIAGNOSTIC TESTING
Sensitivity for LEMS
Electrodiagnostic studies are often considered the gold standard for the diagnosis of neuromuscular disorders, including LEMS, due to their sensitivity for detecting impairments in neuromuscular transmission.6,22
What tests are utilized?
Electrodiagnostic testing typically includes4,23:
- Nerve conduction studies, including repetitive nerve stimulation (RNS)
- Post-exercise facilitation
- Single fiber electromyography (SFEMG)
- SFEMG may be slightly more sensitive than RNS, but it does not distinguish between myasthenia gravis (MG) and LEMS
Hallmarks of LEMS
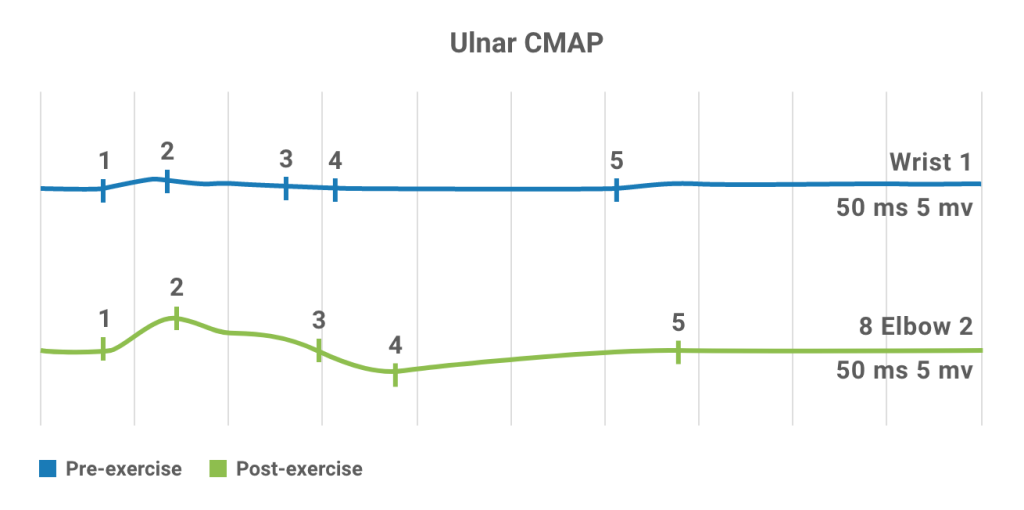
The classic electromyography demonstration of a neuromuscular junction transmission defect is the documentation of a decremental response of the compound muscle action potential (CMAP) to RNS4.
In LEMS, RNS abnormalities include4:
- Low CMAP amplitudes
- Decrement ≥10% at low frequency (2-5 Hz)
- Increment >100% after maximum voluntary contraction or at high frequency (50 Hz)
Post-exercise potentiation
- Post-exercise facilitation is often performed first to exclude or confirm a potential diagnosis of LEMS, because this test is simple and relatively painless24
- An increase in CMAP amplitude greater than 100% demonstrated immediately after a brief, 10- to 30-second exercise is a classic electrodiagnostic hallmark of LEMS4,25
- Among patients who do not demonstrate the diagnostic incremental value after 10-second exercise, high-frequency RNS is needed24
- Post-exercise stimulation has a sensitivity of 84%-96% and is 100% specific for LEMS4
Nerve conduction studies in a patient with LEMS. Baseline CMAP is very small (top).
Following brief exercise, >100% increment is observed in CMAP (bottom), characteristic of LEMS.26
VISUALIZING THE DIAGNOSTIC PROCESS
LEMS diagnosis and oncological screening algorithm27
Start with the Signs & Symptoms tab and then follow the diagnostic process from Signs & Symptoms through Cancer Screening.
SIGNS & SYMPTOMS
Observation of hallmark LEMS signs and symptoms—including proximal muscle weakness, autonomic dysfunction (eg, dry mouth, impotence) and diminished or absent tendon reflexes—should prompt suspicion of LEMS and testing for the presence of anti-VGCC antibodies. Anti-VGCC antibodies are present in virtually all LEMS cases.4
ANTIBODY TESTING
High titers of anti-VGCC antibodies (>30 pmol/L*) indicate a positive diagnosis of LEMS.21 Based on patient history and risk factors, oncologic screening should be considered.4 Evaluate the benefit of treatment of LEMS symptoms with an approved therapy.
Low titers (<30 pmol/L*) suggest a negative or normal diagnosis.21 However, EMG testing should be considered to rule out the possibility of seronegative LEMS. Up to 15% of patients with LEMS have undetectable anti-VGCC antibody levels.4
ELECTRODIAGNOSTIC TESTING (EMG)
Increment on high-frequency nerve stimulation and post-exercise facilitation can provide confirmatory evidence of a diagnosis of LEMS.4 Based on patient history and risk factors, oncologic screening may be considered.27
EMG results consistent with LEMS, together with a negative antibody panel, suggest a diagnosis of seronegative LEMS. Based on patient history and risk factors, oncologic screening may be considered.27
Although a clinical diagnosis of LEMS is still possible without a positive EMG result and a negative anti-VGCC antibody panel, the patient is unlikely to have LEMS. Consider investigating another cause for the patient’s symptoms.
CANCER SCREENING
Because up to 60% of LEMS cases are associated with underlying tumor—typically small cell lung cancer (SCLC)—consider screening newly diagnosed LEMS patients for cancer.4,29 Common screening methods may include CT and PET scan, but other test methods may be considered.4
Consult with the oncologist to determine if symptomatic treatment of the patient’s LEMS symptoms would be beneficial.
If no cancer is found, symptomatic treatment should be initiated and follow-up cancer screening should be performed every 6 months for 2 years.
LEMS is a clinically important early indicator of possible cancer; therefore, a LEMS diagnosis should immediately prompt rigorous oncological screening and surveillance.
—Schoser et al, 201727
ASSESSING LEMS VIA TELEHEALTH
Benefits of telemedicine for LEMS patients
Minimizes exposure to viruses in a potentially immunocompromised patient group
Eliminates barriers to care for patients who may have transportation or mobility issues
Makes it easier and safer for the patient’s caregiver to be present and assist during the exam
Household items patients and/or caregivers can use to facilitate a neuromuscular exam
A small flashlight, to assess dilation of the patient’s pupils
A small hairbrush, to tap joints and check the patient’s reflexes
A dining room chair, to assess the patient’s difficulty in rising and sitting
Patient history may reveal signs of LEMS
Conversation is the first diagnostic tool in LEMS and is the easiest to use via telemedicine. Begin the evaluation with a thorough medical and family history, and you may quickly uncover signs that point to LEMS as a suspect.
CONVERSATION KEYS
- Learn more about the patient’s pattern of muscle weakness, including details regarding4:
- Whether their limb weakness is proximal or distal
- The severity and duration of symptoms
- The onset and persistence of weakness
- Ask about common autonomic symptoms such as dry mouth, constipation, orthostatic hypotension, and impotence4
- Find out if they’re having trouble climbing stairs or rising from a chair3
- Probe for symptoms that suggest an unrecognized malignancy (eg, unexplained weight loss, persistent cough)4,12,28
- If LEMS is suspected, ask questions to distinguish the symptoms from those of myasthenia gravis (ie, presence of double vision, ptosis, and weakness following exercise)1,13,29
Simple LEMS tests easily conducted via telehealth
Assess mobility by observing the patient rising from a chair and/or getting up from the floor (with the help of a caregiver or family member)
Observe the patient’s gait by watching them walk away from the screen and back
Test reflexes by having a family member present who can tap on the patient’s knees to reveal signs of hyporeflexia or areflexia. Have the patient flex/exercise the quadriceps muscle, and then recheck the patient’s knee reflex. Improved reflex post-exercise could be indicative of LEMS4
Evaluate upper-leg strength by having the patient lift up a knee and asking the caregiver or a family member to push it down to see if there is resistance
Examine the pupils by asking the patient to move close to the camera, and have the caregiver shine a flashlight in their eyes, so that you may identify any evidence of a cholinergic defect such as blurred vision

